
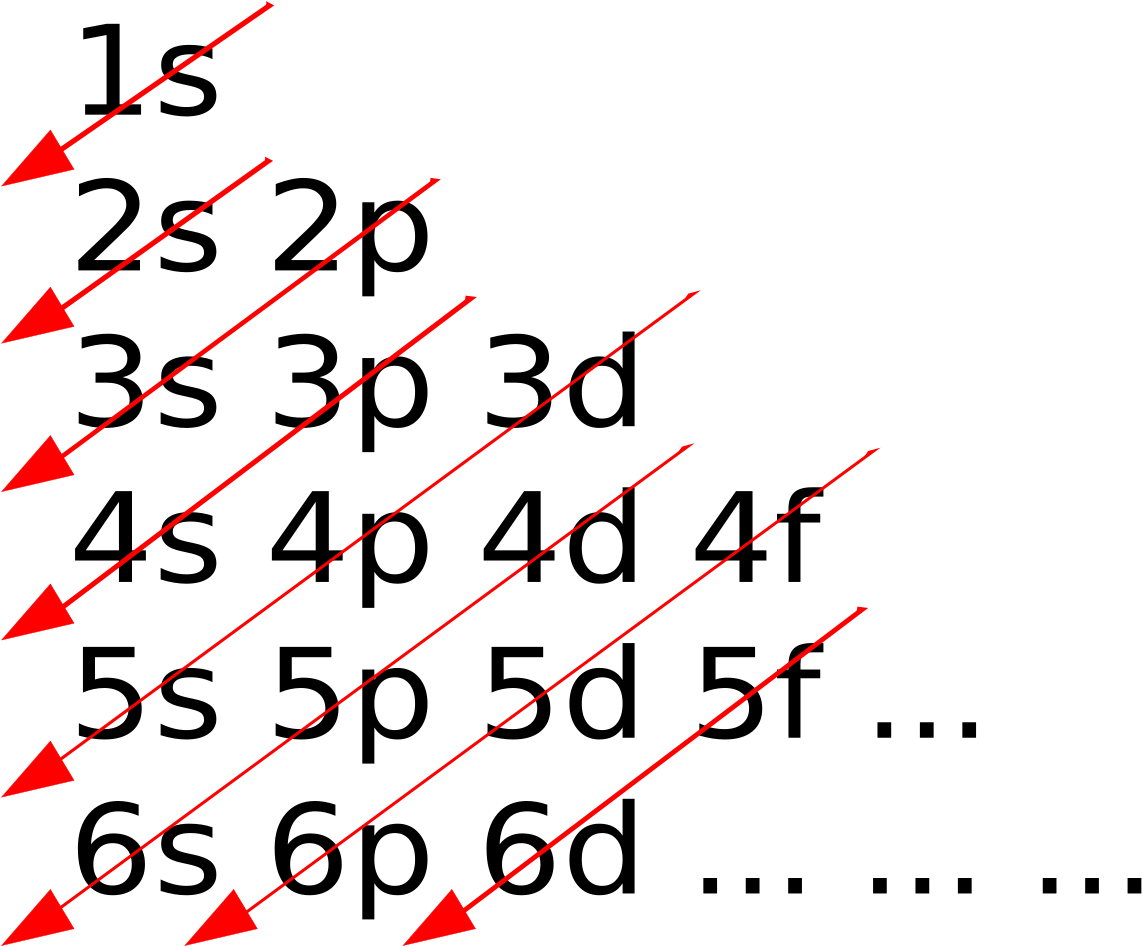
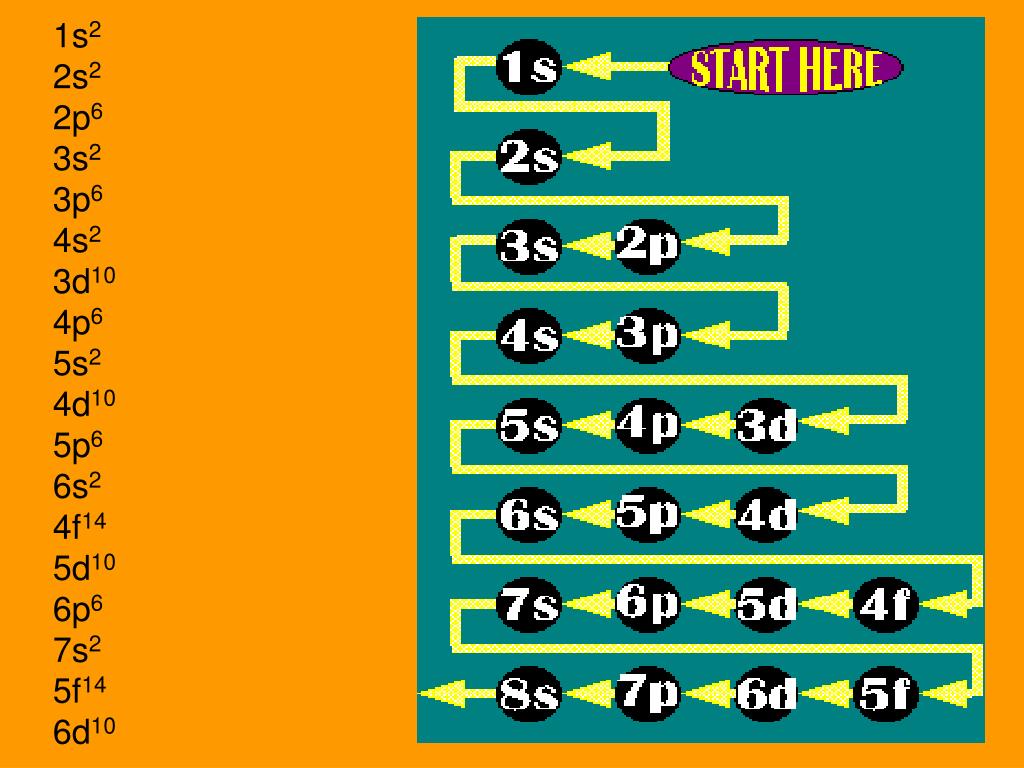
Use the electron configuration mnemonic to complete the table below
Question Electronic configuration of first 30 elements with table full explanation Solution H (Hydrogen) 1s1 He (Helium) 1s2 Li (Lithium) 1s2 2s1 Be (Beryllium) 1s2 2s2 B (Boron) 1s2 2s2 2p1 C (Carbon) 1s2 2s2 2p2 N (Nitrogen) 1s2 2s2 2p3 O (Oxygen) 1s2 2s2 2p4 F (Fluorine) 1s2 2s2 2p5 Ne (Neon) 1s2 2s2 2p6 Na (Sodium) 1s2 2s2 2p6 3s1

Which element has the electron configuration of 1s2 2s2 2p6 3s2 3p6 3d5
Which element has the electron configuration of 1s2 2s2 2p6 ? Wayne Breslyn 728K subscribers Join Subscribe Subscribed 383 54K views 3 years ago To figure this out the element with the electron.
Wiswesser S Wikipedia 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6
1s2 2s2 2p6 3s2 3p5: Argon: 1s2 2s2 2p6 3s2 3p6: Potassium: 1s2 2s2 2p6 3s2 3p6 4s1: Calcium: 1s2 2s2 2p6 3s2 3p6 4s2: Mr. Vissers. Send e-mail; This activity was created by a Quia Web subscriber. Learn more about Quia: Create your own activities.
1s2 2s2 2p6 3s2 3p6 Elektronlar Atomun Etrafında Nerede Bulunuyor
The electron configuration of palladium is 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s0 4d10. Palladium belongs to group number 10 and period number 5 of the periodic table. Palladium is known for its resistance to tarnishing and non-toxic nature. It is a member of the platinum group elements and exhibits high reactivity.

1s2 2s2 2p6 3s2 3p6 4s1 3d5 KoltenkruwHernandez
1s2 2s2 2p6 3s2 3p6 3d10 4s1. For the Cu+ ion we remove one electron from 4s1 leaving us with: 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10. For the Cu2+ ion we remove a total of two electrons (one from the 4s1 and one form the 3d10) leaving us with. 1s 2 2s 2 2p 6 3s 2 3p 6 3d 9

1s2 2s2 2p6 3s2 What is 1s2 2s2 2p6 3s2 ?What element has the
The electron configuration of sulfur is 1s2 2s2 2p6 3s2 3p4. A step-by-step description of how to write the electron configuration for Sulfur (S). Explain It To A Child. The most common configuration of electrons for sulfur is 1s2 2s2 2p6 3s2 3p4. This means that the sulfur atom has two electrons in the first energy level, two electrons in the.

Which element has the electron configuration of 1s2 2s2 2p6 3s2 3p6 3d1
Key Questions How do electron configurations correspond to the periodic table? When looking at electron configuration, your fill order of electrons is: 1s 2s 2p 3s 3p 3d 4s 4p 4d 4f 5s etc. Group 1A (1), the alkali metals all end is s1. What period the element is in determines the 1st number.
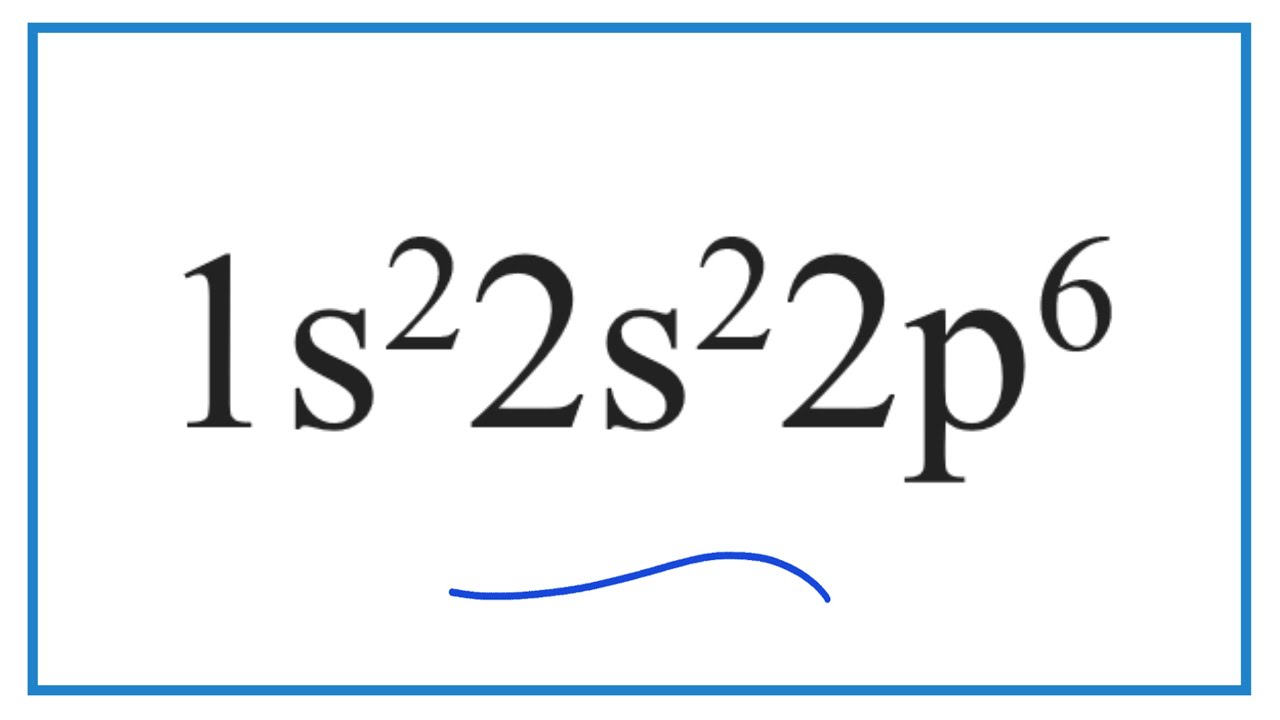
Which element has the electron configuration of 1s2 2s2 2p6 ? YouTube
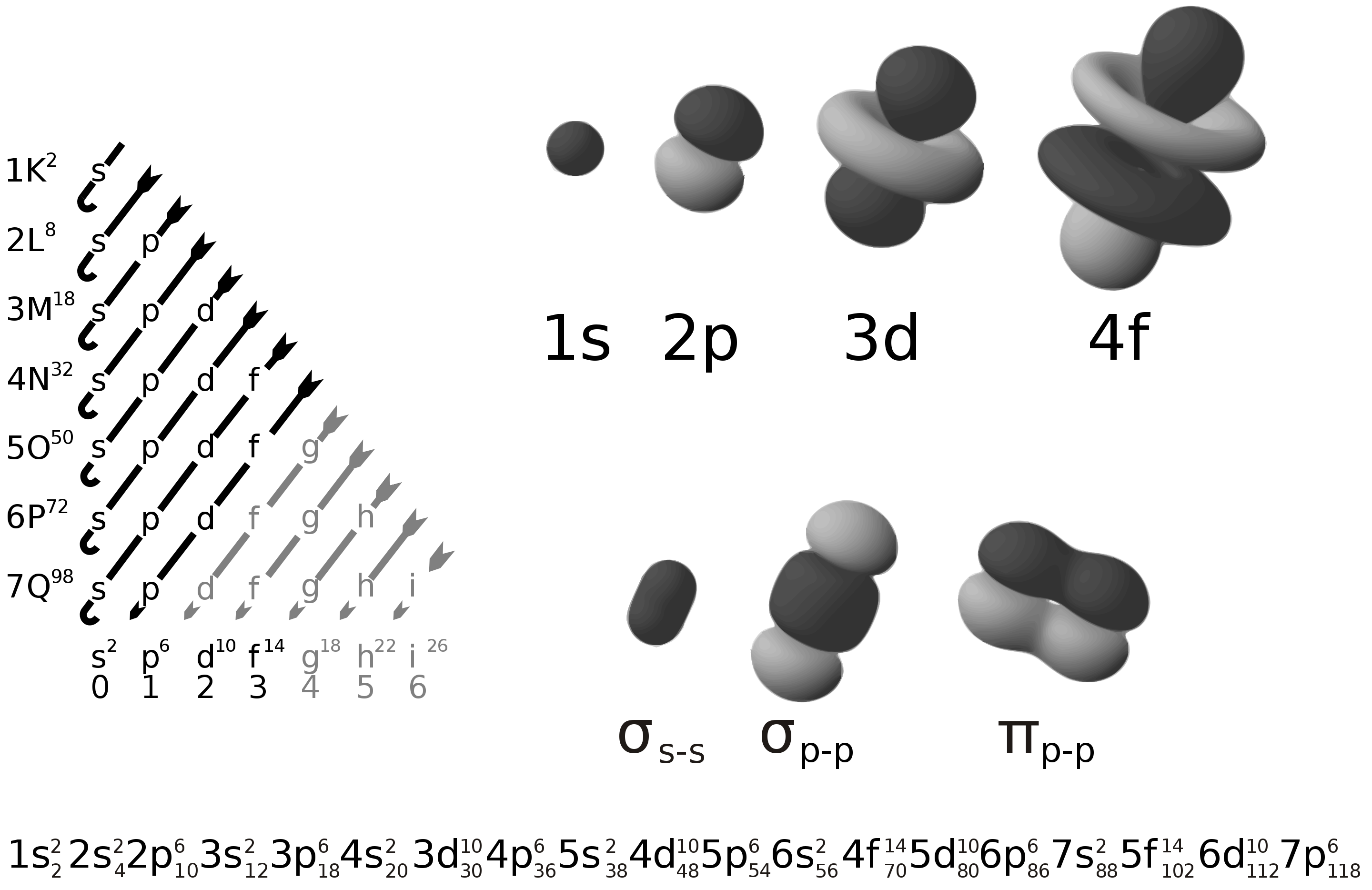
1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p6 4f14 5d10 6s2 6p6 5f14 6d10 7s2 7p6: Periodic Table of Elements with. Electron Configuration Full TrendsIn the below periodic table you can see the trend of . Electron Configuration Full. For facts, physical properties, chemical properties, structure and atomic properties of the specific element.
PPT Electron Configuration Notes PowerPoint Presentation, free
Like you can write 1s2 2s1 out as [He] 2s1, which basically means the electron configuration of He (the previous row's noble gas), and then whatever else is outside the square brackets. It doesn't save much space here, but imagine if you were writing the electron configuration for say iron out, it takes much less space to write out [Ar] 4s2 3d6 than to write 1s2 2s2 2p6 3s2 3p6 4s2 3d6

1s2 2s2 2p6 Which element has the electron configuration of 1s2 2s2
atomic physics (or other physical structure) in [1] For example, the electron configuration of the , meaning that the 1s, 2s and 2p subshells are occupied by 2, 2 and 6 electrons respectively. Electronic configurations describe each electron as moving independently in an orbital, in an average field created by all other orbitals.

Which element has the electron configuration of 1s2 2s2 2p6 3s2 3p6 3d5
Half-filled and fully filled subshell have got extra stability. Therefore, one of the 4s2 electrons jumps to the 3d5 so that it is half-filled (see video below). This give us the (correct) configuration of: 1s2 2s2 2p6 3s2 3p6 3d5 4s1

Which element has the electron configuration of 1s2 2s2 2p6 3s2 3p3
This electron configuration calculator will instantly show you the distribution of electrons in the orbitals of any periodic element you choose. Typically, you need at least 8 steps to determine the electron configuration, starting with finding the atomic number by looking at the list of orbitals and understanding the notation.
1s2 2s2 2p6 astonishingceiyrs
The total number of electrons present in the given electronic configuration is 1s22s22p6 = 2+2+6= 10. As, number of electrons = numbers of protons = 10. Hence, the given electronic configuration is of Neon. Suggest Corrections. 4.

1s2 2s2 2p6 3s2 3p6 4s2 3d10 Element LeonhasHines
1s2 2s2 2p6 -> neon (Ne). By the way, it's not electricity configuration - it's electronic configuration. The root word is "electron", the orbitals of which is described by 1s2 2s2 2p6. As for the best way to determine the element purely based on its electronic configuration, it is difficult for elements beyond 18 which is argon (Ar). For elements below proton number 18, if the electronic.

Which element has the electron configuration of 1s2 2s2 2p6 3s2 3p6 3d3
So, 1s2 2s2 2p6. I showed you how you could look at the periodic table and kind of run through these electron configurations. For example, this would be 1s1, 1s2 and then 2s2 would be here and then we had six. So 2p6 brings you all the way over to neon. And so for an electron configuration for the elements in the third period, so this would be.

1s2 2s2 2p6 astonishingceiyrs
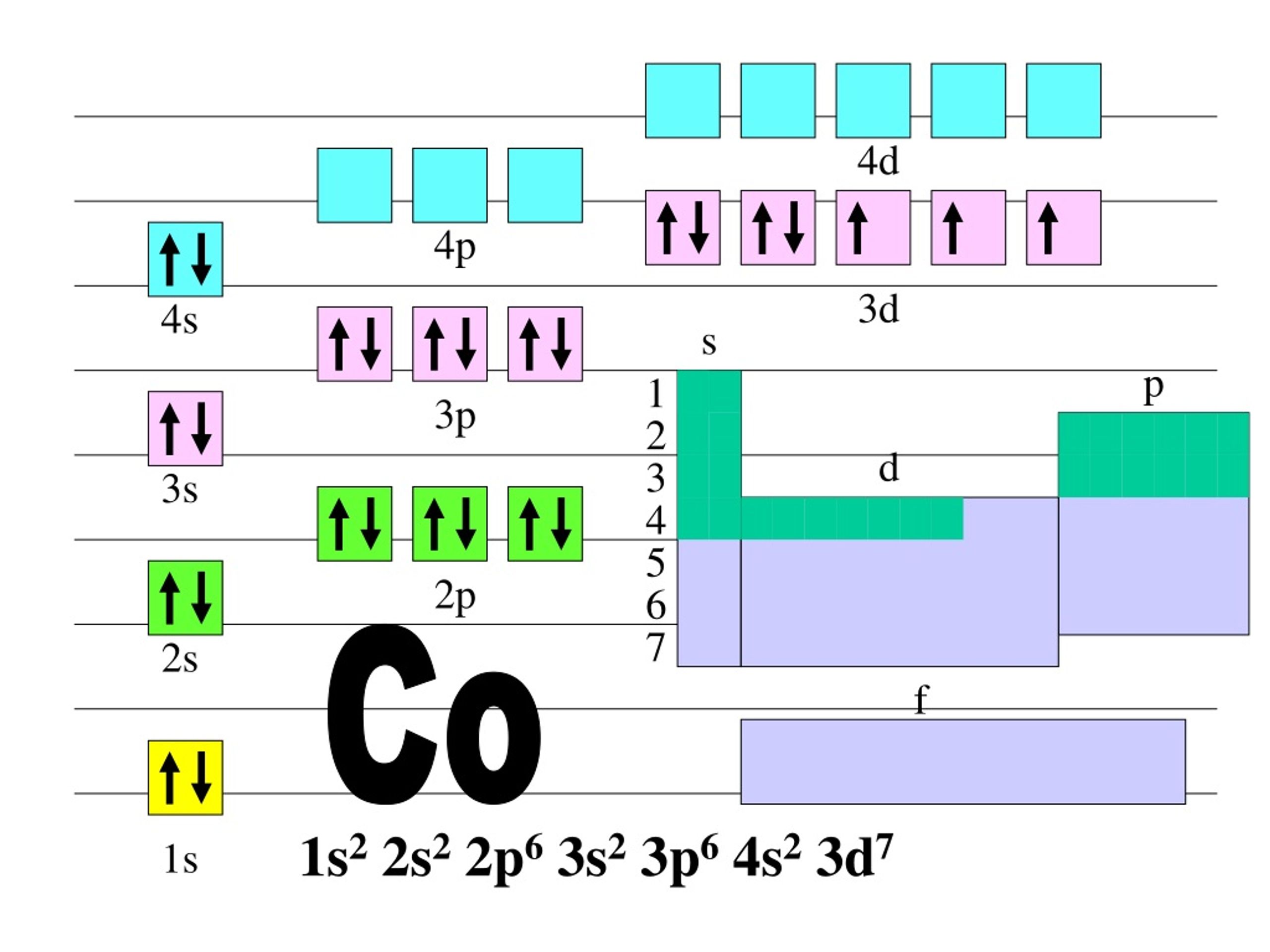
PROBLEM 3.1.12 3.1. 12. In one area of Australia, the cattle did not thrive despite the presence of suitable forage. An investigation showed the cause to be the absence of sufficient cobalt in the soil. Cobalt forms cations in two oxidation states, Co 2+ and Co 3+. Write the electron structure of the two cations. Answer.