Top No3 Lewis Structure Molecular Geometry Bond Angles Background GM
Drawing the Lewis Structure for NO 3- ( Nitrate Ion) Nitrates (salts with NO 3-) are frequently used in agriculture as a fertilizer. This is in part to their high solubility in water. There are 24 valence electrons available for the Lewis structure for NO 3-. Try to draw the NO 3- Lewis structure before watching the video.
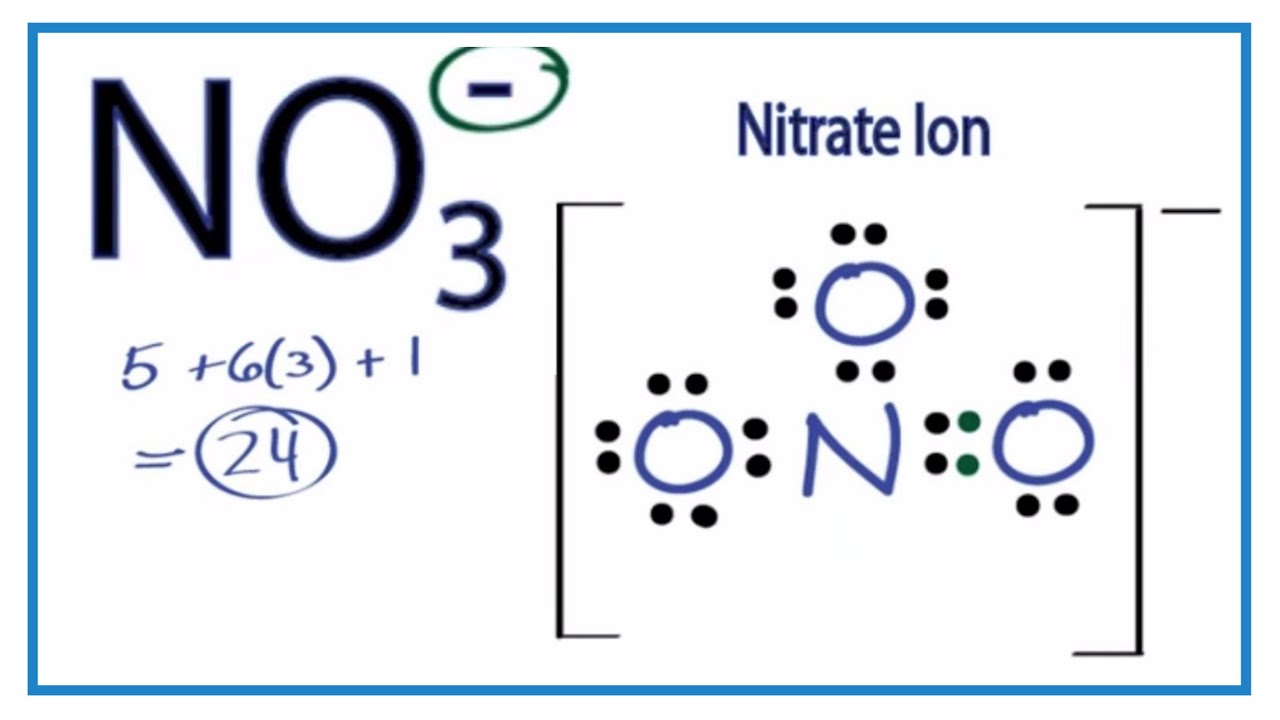
NO3 Lewis Structure How to Draw the Lewis Structure for NO3 YouTube
Lewis structure of a water molecule. Lewis structures - also called Lewis dot formulas, Lewis dot structures, electron dot structures, or Lewis electron dot structures (LEDs) - are diagrams that show the bonding between atoms of a molecule, as well as the lone pairs of electrons that may exist in the molecule. A Lewis structure can be drawn for any covalently bonded molecule, as well as.

Chemistry Class 11 NCERT Solutions Chapter 4 Chemical Bonding and
Follow these simple steps to draw Lewis dot structures: Draw the atoms on paper and put dots around them to represent valence electrons of the atom. Be sure to have the correct number of electrons. If the species is an ion, add or subtract electrons corresponding to the charge of the ion.
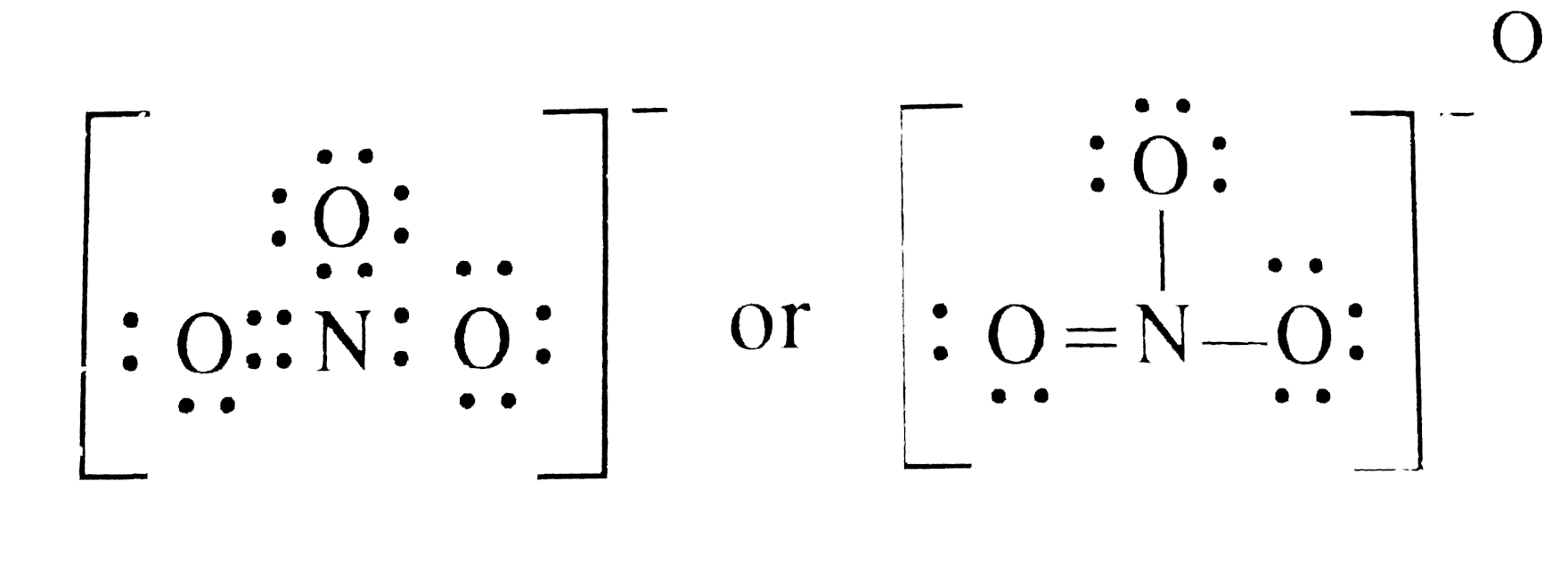
How To Draw The Lewis Dot Structure For No3 Nitrate Ion
A dot structure is any representation of atoms/molecules using dots for electrons. And a Lewis diagram (or Lewis structure or Lewis dot structure) is a type of dot structure created by the chemist Gilbert N. Lewis which is most commonly used in chemistry nowadays. There's a slight difference, but they effectively mean the same thing.

NO3 Molecular Geometry / Shape and Bond Angles YouTube
A Lewis electron dot diagram (or electron dot diagram, or a Lewis diagram, or a Lewis structure) is a representation of the valence electrons of an atom that uses dots around the symbol of the element. The number of dots equals the number of valence electrons in the atom. These dots are arranged to the right and left and above and below the.
No3 Dot Structure
The NO3- Lewis structure represents the nitrate ion, which consists of one nitrogen atom and three oxygen atoms. The central nitrogen atom forms one double bond and two single bonds with the three surrounding oxygen atoms.

No3 Lewis Structure
Lewis structure of NO3- ion (nitrate ion) contains one double bond and two single bonds between the Nitrogen (N) atom and Oxygen (O) atoms. The Nitrogen atom (N) is at the center and it is surrounded by 3 Oxygen atoms (O). Let's draw and understand this lewis dot structure step by step.

How to Draw the Lewis Dot Structure for Sr(NO3)2 Strontium nitrate
Lewis structure of NO 3- ion is drawn step by step in this tutorial. Total valence electrons of nitrogen and oxygen atoms and negative charge are considered to draw the NO 3- lewis structure. You will every fact of drawing lewis structures from this tutorial which will help you to draw more lewis structures in the future.

How To Draw The Lewis Dot Structure For No3 Nitrate Ion
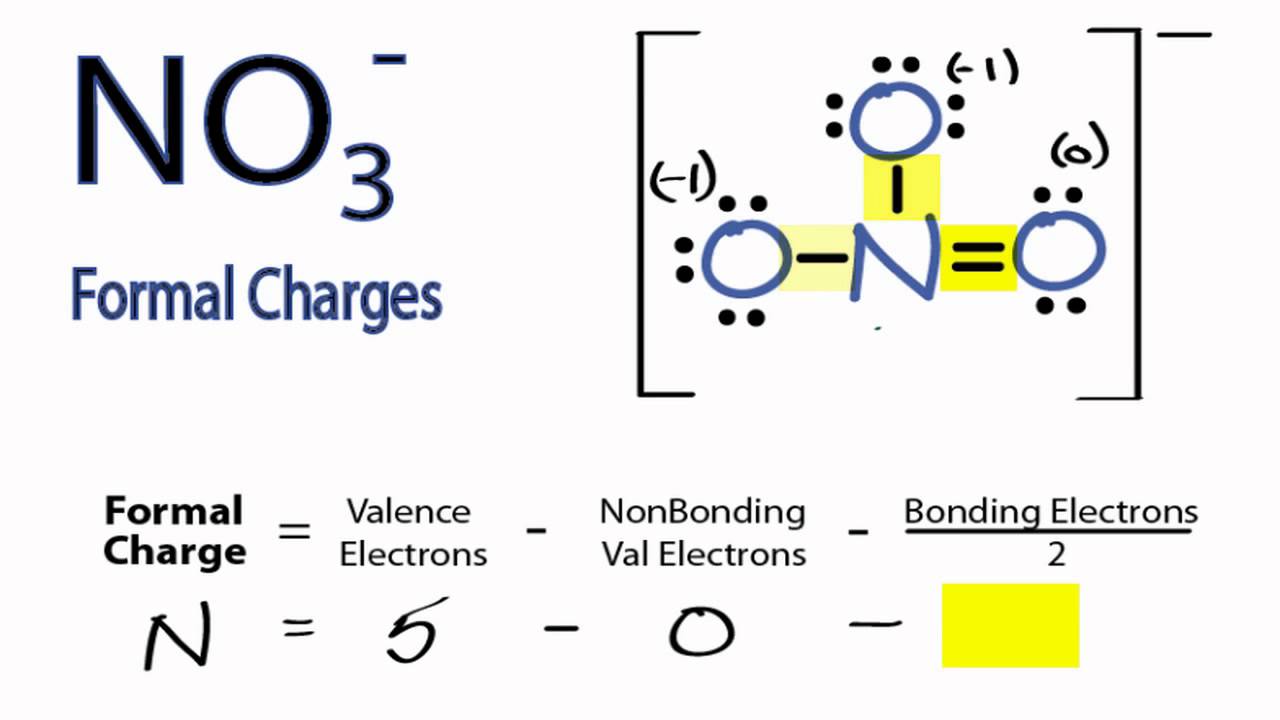
in the lewis structure for nitrate ion, NO3-, what is the total number of reasonable resonance structures that can be drawn? Three reasonable resonance structures can be drawn. Each of those resonance structures, two oxygen atoms have charges (each one has -1) and nitrogen atom has a +1 charge.
no2 bond order
NO 3- Back 70 More Lewis Dot Structures The nitrate ion cannot be satisfactorily represented by just one Lewis Dots structure. All the bonds are the same length and must be thought of as a hybrid of multiple resonance structures. Lewis Dot Structure of NO3- (Nitrate Ion) Watch on from http://treefrog.fullerton.edu/chem/LS/NO3neg1LS.html

NO3 Lewis Structure, Molecular Geometry, and Hybridization
Lewis Dot Structure of NO3- (Nitrate Ion) kentchemistry.com 25.1K subscribers Subscribe Subscribed 294K views 12 years ago Every Video I quickly take you through how to draw the Lewis.

Lewis Structure NO3 plus dipoles, shape, angles, resonance and formal
Contents show Construction of NO3 Lewis Dot Structure 1. In the ion NO3, there is 1 atom of nitrogen and 3 atoms of oxygen. It also has one negative charge. 2. Nitrogen and oxygen belong to periods 5A and 6A groups respectively in the periodic table. Hence, oxygen has 6 and nitrogen has 5 valence electrons in their outer shell.

SOLVED Draw the Lewis Dot Structure for nitrate, NO31
How to draw lewis structure of NO3-? The Lewis structure of a nitrate [NO3]- ion consists of a nitrogen (N) atom and three oxygen (O) atoms. The nitrogen (N) atom is present at the center of the molecular ion, while three oxygen (O) atoms occupy terminal positions, one on each side.

Lewis dot structure of NO3 ion Nitrate ion lewis structure YouTube
GENERAL TERMS FOR LEWIS DOT STRUCTURES: 1. Dot • one dot represents one valence electron (found on odd-electron particles). 2. Pair of Dots •• a pair of dots represents a nonbonding (lone) pair of electrons that are not involved in a covalent bond and "belong to" only one atom. 3. Dash each dash represents two electrons that are shared between two atoms as a covalent bond.

Lewis dot structure of the nitrate ion NO3 YouTube
How to Draw the Lewis Dot Structure for NO3 - (Nitrate ion) - YouTube A step-by-step explanation of how to draw the NO3- Lewis Dot Structure (the Nitrate ion).For the NO3 - structure.

Lewis structure of NO3 (Nitrate ion)Draw the Lewis dot structure of
The Lewis structure for NO 3- (Nitrate Ion) comes up quite often in chemistry. Be sure to put brackets, along with a negative sign, around the NO 3- Lewis structure when you are done to show that it is an ion with a negative charge. NO 3- has a total of 24 valence electrons. NO3- Lewis Structure: How to Draw the Lewis Structure for NO3- Watch on