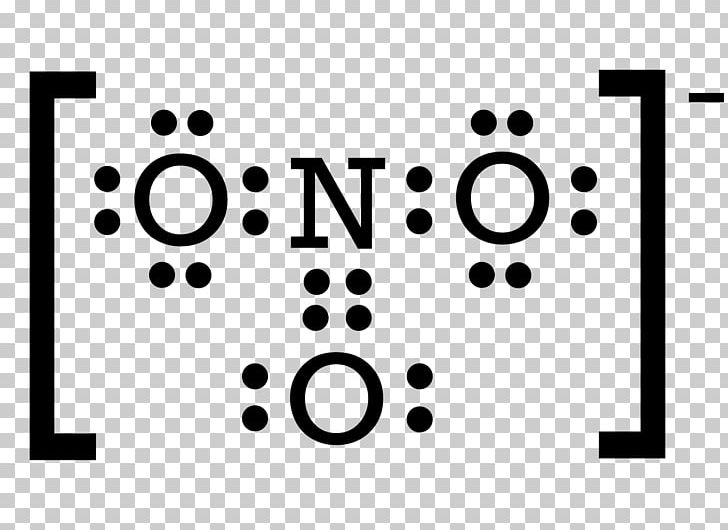
Lewis Structure Nitrite Sodium Nitride Covalent Bond Nitrate PNG, Clipart, Auto Part, Black
A step-by-step explanation of how to draw the NO3- Lewis Dot Structure (Nitrate ion).For the NO3- structure use the periodic table to find the total number o.

Write the various steps involved in the Lewis structure for nitrate `(NO_(3)^())` ion
The nitrite ion, which is NO2 (-1), has two oxygen atoms connected to a central nitrogen atom. To satisfy the octet on nitrogen, exactly ONE of the oxygens needs to be double-bonded to it. But.

The chemical formula of nitrite infographics Vector Image
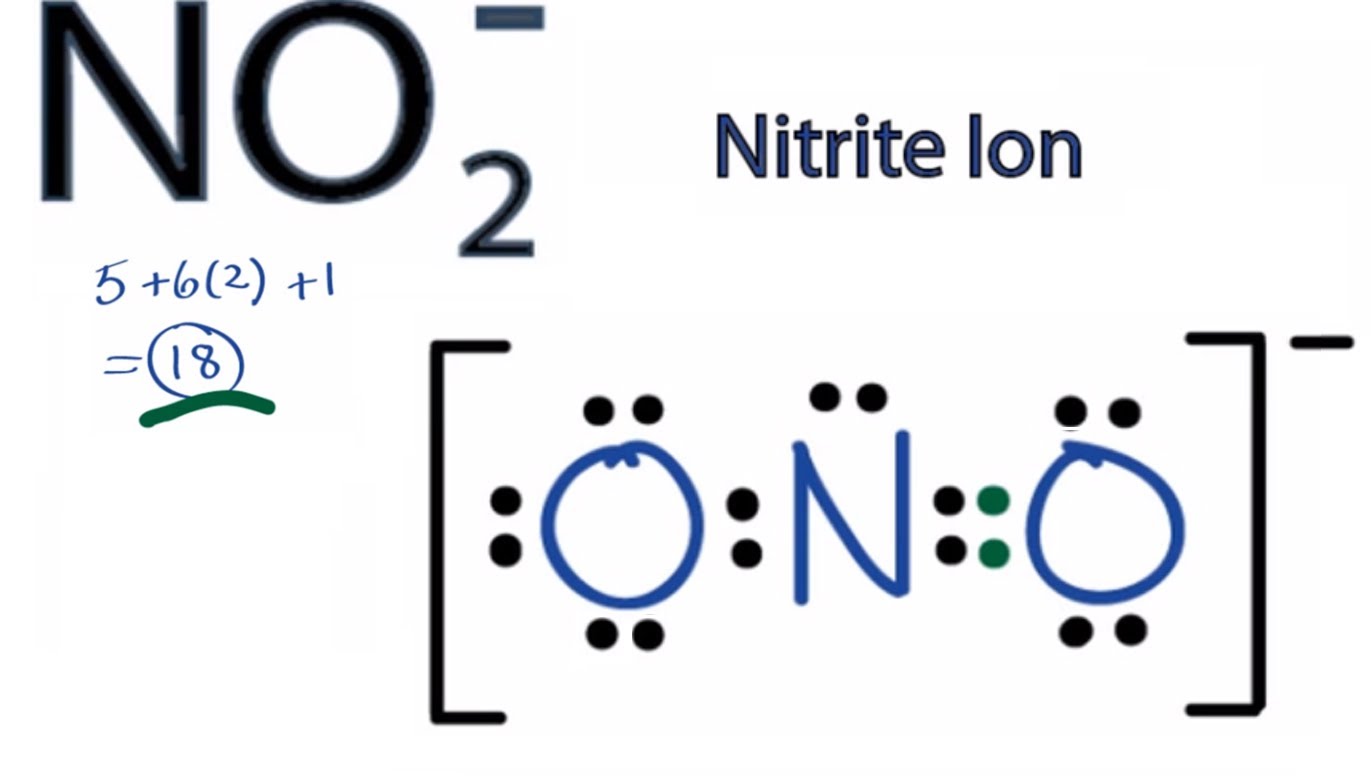
Lewis Structure for NO 2- (Nitrite ion) Lewis structure of NO 2- ion is drawn in this tutorial. Total valence electrons of nitrogen and oxygen atoms and negative charge also should be considered in the drawing of NO 2- lewis structure. Now, we are going to learn, how to draw this lewis structure. Steps of drawing NO 2- lewis structure

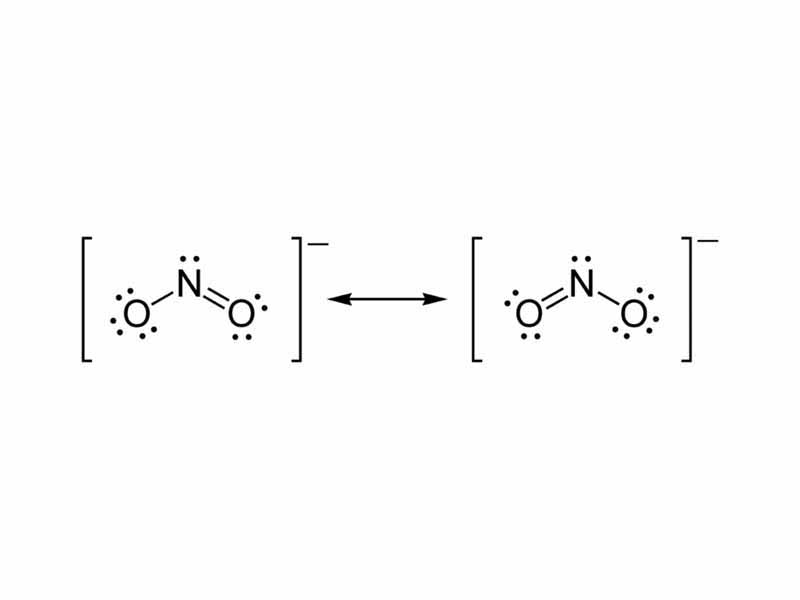
Draw the resonance structures of nitrite ion. Quizlet
Structure Molecular Formula NO2- Synonyms nitrite Nitrite Ion 14797-65- Nitrite anion Nitrous acid, ion (1-) View More. Molecular Weight 46.006 g/mol Computed by PubChem 2.2 (PubChem release 2021.10.14) Dates Create: 2005-03-26 Modify: 2023-12-30 Description Nitrites, inorganic, n.o.s. appears as colorless solutions or crystalline solids.

Write the Lewis structure of the nitrite ion, NO2^
A step-by-step explanation of how to draw the NO3- Lewis Dot Structure (the Nitrate ion).For the NO3 - structure use the periodic table to find the total num.
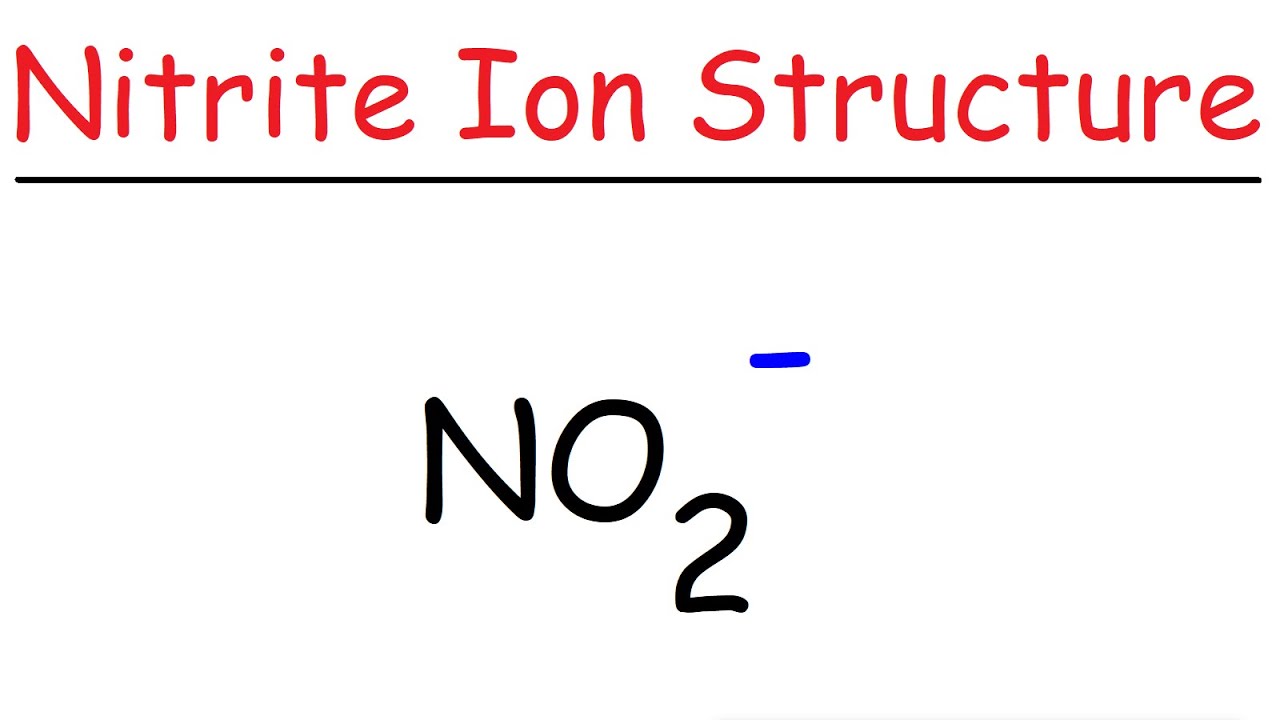
NO2 Lewis Structure Nitrite Ion YouTube
Drawing the Lewis Structure for NO 3-(Nitrate Ion). Nitrates (salts with NO 3-) are frequently used in agriculture as a fertilizer.This is in part to their high solubility in water. There are 24 valence electrons available for the Lewis structure for NO 3-.. Video: Drawing the Lewis Structure for NO 3-. n

Write the Lewis structure of the nitrite ion, NO2^
This chemistry video tutorial explains how to draw the lewis structure of NO2-, the Nitrite ion.Chemistry - Basic Introduction: https://ww.

nitrite lewis structure Dot diagram for sodium ion viral fr
This chemistry video tutorial explains how to draw the lewis structure of the nitrate ion NO3-.Chemistry - Basic Introduction: https://www.
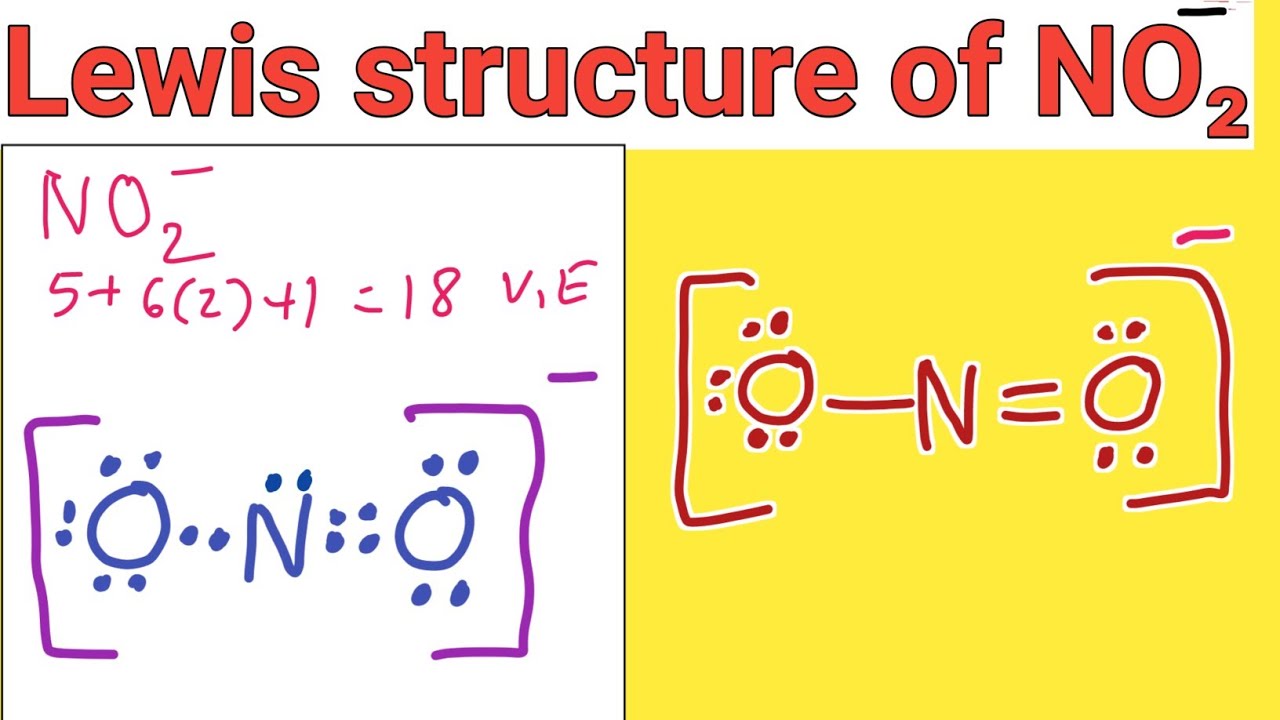
Lewis structure of NO2 (Nitrite ion) Trick to draw Lewis dot structure for NO2 ion YouTube
Lewis Structure Tutorial A step-by-step explanation of how to draw the NO2 - Lewis Dot Structure (Nitrite ion).For the NO2 - structure use the periodic table to find the total number.

write the Lewis structure of nitrite ion no2 Brainly.in
Lewis Structure (6): Nitrite ion - YouTube A simple way to draw the preferred Lewis electron dot structure for the nitrite anion. I simply draw the valence electrons around each atom and.

nitrite formula Sodium nitrite facts, formula, properties, uses viral fr
Put lone pairs on atoms Stability of lewis structure - Check the stability and minimize charges on atoms by converting lone pairs to bonds. Drawing correct lewis structure is important to draw resonance structures. In another tutorial, we learn how to draw resonance structures of nitrate ion.

Orbitali ibridi
If you are thinking about it like literally adding O to the nitrite ion then yes, the two electrons the oxygen receives are from nitrogen. Imagine moving the nitrogen lone pair to the oxygen to give it a full octet and forming a single bond in the process. You will notice that the Lewis structures will match the "organiker" structures of Uncle.

Nitrite anion chemical structure skeletal formula Vector Image
Example: What is the Lewis structure for the nitrite ion (NO 2 −)? Answer: Nitrogen is the least electronegative atom and should be the central atom. After counting the valence electrons, we have a total of 17 [5 from nitrogen + 2(6 from each oxygen)] = 17.
Science Image Archive for Teachers
What is the Lewis structure of nitrite ion, N O− 2? Chemistry Covalent Bonds Drawing Lewis Structures 1 Answer anor277 Sep 9, 2017 Well, we gots 5(N) + 2 × 6(O) + 1(negative charge) valence electrons to distribute.. Explanation: And thus,. O = ..
How to count formal charge in Nitrite Ion? Chemistry Science Forums
The nitrite ion cannot be satisfactorily represented by just one Lewis Dots structure. All the bonds are the same length and must be thought of as a hybrid of multiple resonance structures. When constructed the NO bond has a bond order of 1.5. It is stronger than a single bond but weaker than a double. Lewis Dot Structure of NO2- (Nitrite Ion)

Lewis Structure of NO2(1), the nitrite ion. YouTube
Welcome to Warren Institute! In this article, we will dive into the fascinating world of Chemistry and explore the NO2- Lewis Structure. As aspiring