[Solved] Please slove. Question 52 3 pts The molecular geometry of the
Let's do the Lewis structure for CH2F2: difluoromethane. On the periodic table, Carbon is in group 4, it has 4 valence electrons. Hydrogen, group 1, but we have 2 Hydrogens. Fluorine, 7 valence electrons, we have 2 of those as well, for a total of 20 valence electrons. We'll put the Carbon at the center, and then Hydrogens always go on the outside.

CH2F2 Lewis Structure, Molecular Geometry, Hybridization, and Polarity
∴ Total number of valence electrons available for the CH 2 F 2 Lewis structure = 4 + 1 (2) + 7 (2) = 20 valence electrons [∴ CH 2 F 2 molecule has one carbon, two hydrogen, and two fluorine atoms] 2. Find the least electronegative atom and place it at center
Is CH2F2 Polar or Nonpolar? (Difluoromethane) YouTube
1. The bromine atom has seven valence electrons, and each fluorine has seven valence electrons, so the Lewis electron structure is. Three fluorines are bonded to a central bromine. Each fluorine has three lone pairs, Bromine has two lone pairs. Once again, we have a compound that is an exception to the octet rule. 2.
[Solved] Please slove. Question 52 3 pts The molecular geometry of the
A step-by-step explanation of how to draw the CH2F2 Lewis Dot Structure (Difluoromethane).For the CH2F2 structure use the periodic table to find the total nu.

Ch2F2 Molecular Geometry
Learn to determine if CH2F2 (Dichloromethane) is polar or non-polar based on the Lewis Structure and the molecular geometry (shape).We start with the Lewis.
Ch2f2 Lewis Structure
In the CH 2 F 2 Lewis structure, there are four single bonds around the carbon atom, with two hydrogen atoms and two fluorine atoms attached to it, and on each fluorine atom, there are three lone pairs. CH2F2 Lewis Structure - How to Draw the Lewis Structure for CH2F2 Watch on Contents Steps #1 Draw a rough skeleton structure

Ch3cooh Molecular Geometry
Molecular Model. Structural Formula. CH 2 F 2. difluoromethane . Molecular Model Application loaded..

Top Ch2F2 Molecular Geometry The Latest GM
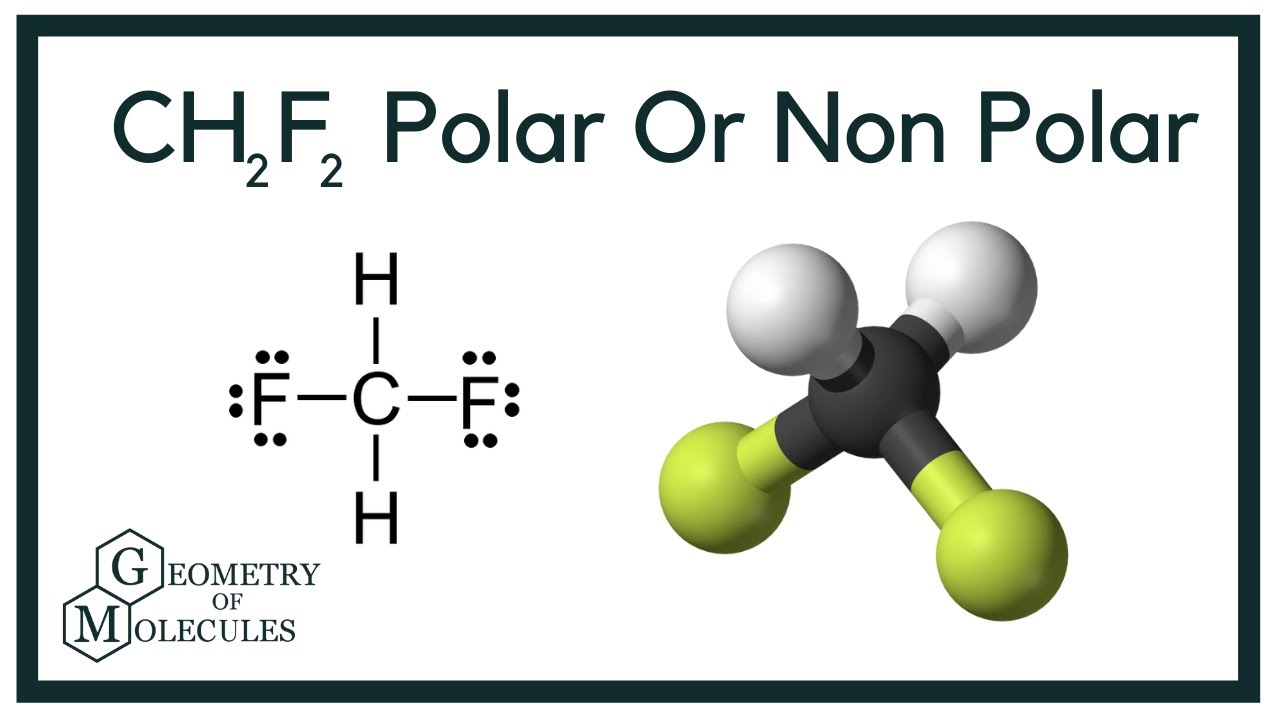
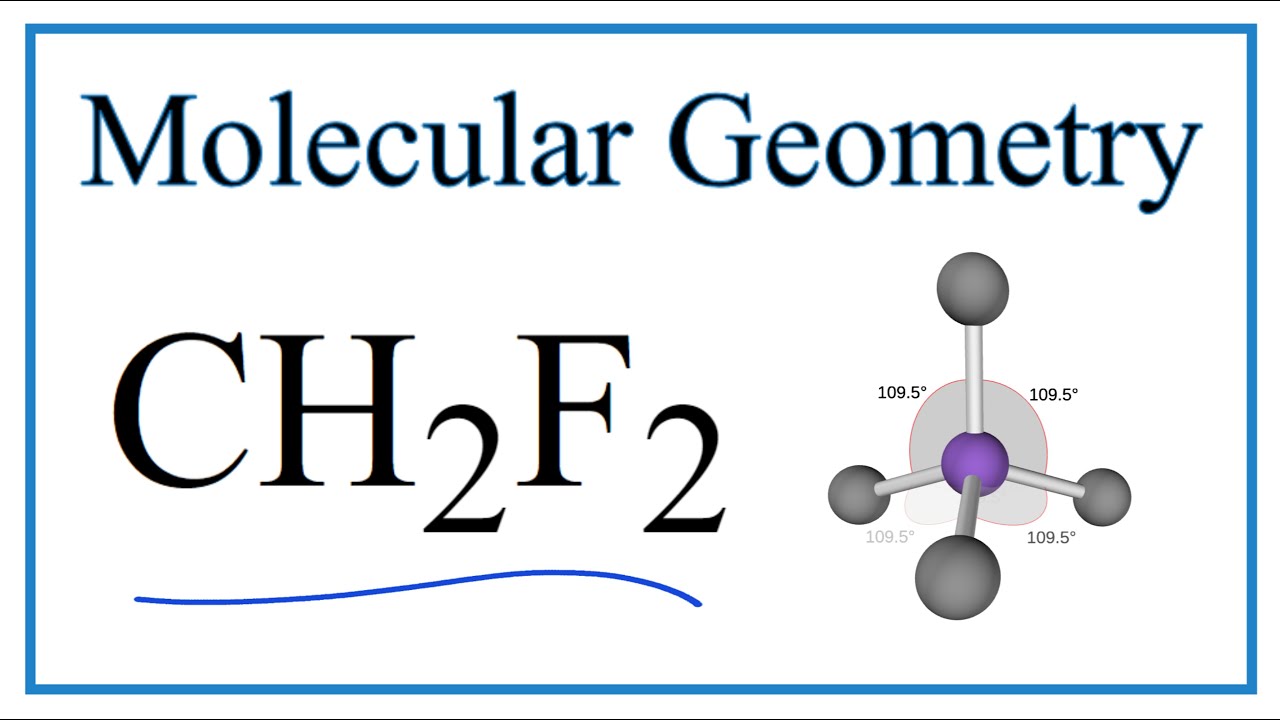
The CH2F2 molecule is classified as a polar molecule. The molecule of difluoromethane (with tetrahedral molecular geometry) is tilted, the bond angles between fluorine, carbon, and hydrogen (H-C-H and F-C-F) are 112.5 and 108.5 degrees.
Solved 1.What Are The Three Types Of Bonding? 2.What Does...
Difluoromethane, also called difluoromethylene, HFC-32 Methylene Fluoride or R-32, is an organic compound of the dihalogenoalkane variety. It has the formula of CH 2 F 2. It is a colorless gas in the ambient atmosphere and is slightly soluble in the water, with a high thermal stability.
CH2F2 Molecular Geometry, Bond Angles (and Electron Geometry) YouTube
CH2F2 is industrially manufactured via the fluorination of Dichloromethane (CH2Cl2). Dichloromethane reacts with Hydrogen Fluoride (HF) in three phases under different conditions. Owing to its thermodynamic properties, Difluoromethane is used as a refrigerant. CFCs and other aerosols have been ruled as damaging to the ozone layer.
Solved Determine the molecular geometry and molecular
An explanation of the molecular geometry for the CH2F2 (Difluromethane) including a description of the CH2F2 bond angles. The electron geometry for the Difluromethane is also provided..

[Solved] CH2F2 Draw the lewis dot structure Dete
Hello Guys!CH2F2 or Dichloromethane has a quite simple structure as it consists of one central carbon atom forming single covalent bonds with two hydrogen an.
Ch2f2 Shape
Step #1: Calculate the total number of valence electrons Here, the given molecule is CH2F2. In order to draw the lewis structure of CH2F2, first of all you have to find the total number of valence electrons present in the CH2F2 molecule. (Valence electrons are the number of electrons present in the outermost shell of an atom).

CH2Br2 Lewis Structure How to Draw the Lewis Structure for CH2Br2
CH2F2 Molecular Geometry The molecular geometry of CH2F2 can be predicted by valence shell electron pair repulsion (VSEPR), a theory given by Sidgwick and Powell. This theory is based on repulsion between valence electrons of the atom.

CH2Cl2 Molecular Geometry Science Education and Tutorials
Figure 5.2.9 5.2. 9: (a) H2O has four regions of electron density around the central atom, so it has a tetrahedral electron-pair geometry. (b) Two of the electron regions are lone pairs, so the molecular structure is bent. Exercise 5.2.3 5.2. 3. The hydronium ion, H 3 O +, forms when acids are dissolved in water.

CH2Cl2 Lewis structure, Molecular geometry, Hybridization, Bond angle
The CH2F2 molecular geometry is a diagram that illustrates the number of valence electrons and bond electron pairs in the CH2F2 molecule in a specific geometric manner.