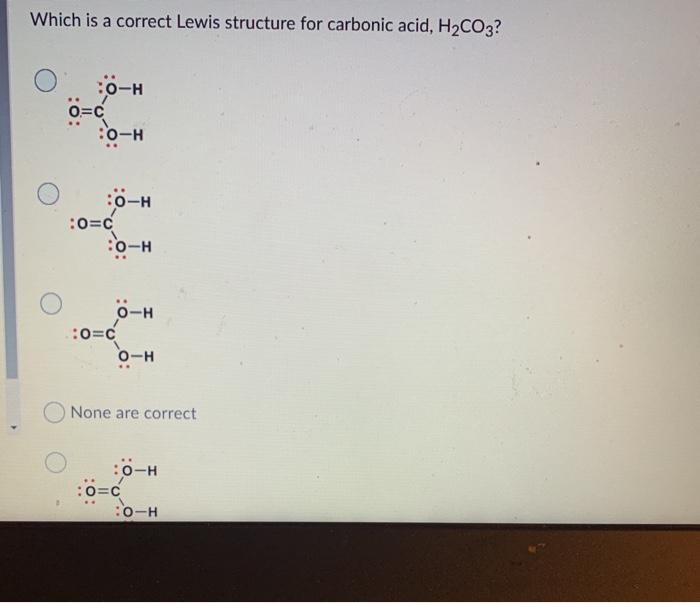
(Get Answer) Which Is A Correct Lewis Structure For Carbonic Acid
Step 1: Figure out how many electrons the molecule must have, based on the number of valence electrons in each atom. When drawing the structure of an ion, be sure to add/subtract electrons to account for the charge. Step 2: Connect the atoms to each other with single bonds to form a "skeleton structure.".

Lewis Structure of H2CO3 How to draw lewis structures for oxoacids
Like ozone, the electronic structure of the carbonate ion cannot be described by a single Lewis electron structure. Unlike O 3, though, the actual structure of CO 3 2 − is an average of three resonance structures. 1. Because carbon is the least electronegative element, we place it in the central position:

8) Covalent bolidnn Carbon dioxide reacts with water to form carbonic
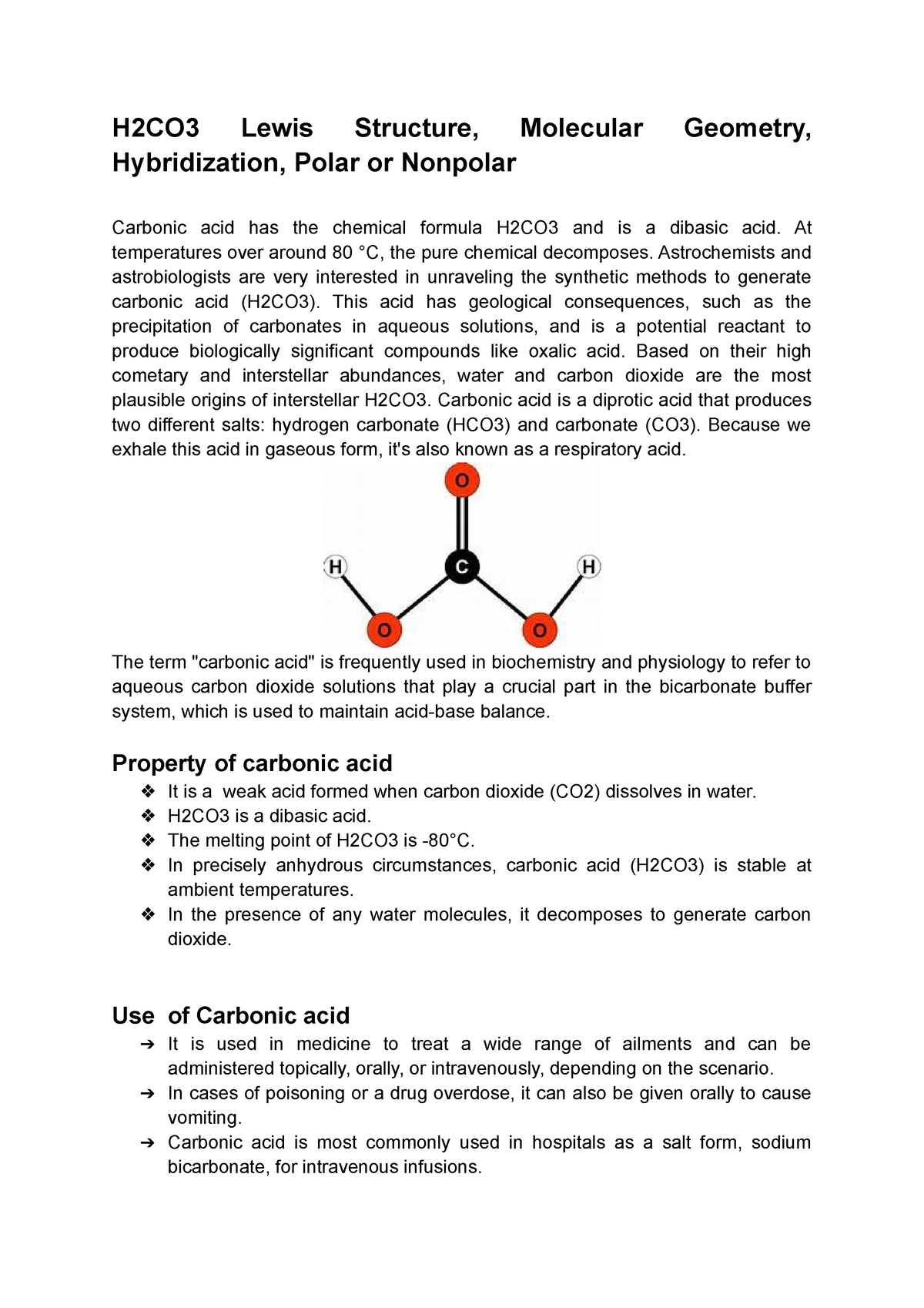
Page Contents show How to draw lewis structure of H2CO3? The Lewis structure of carbonic acid (H2CO3) consists of a carbon (C) atom at the centre. It is double-covalently bonded to an oxygen (O) atom on one side and single-covalently bonded to two hydroxyl (OH) functional groups on the other two sides.
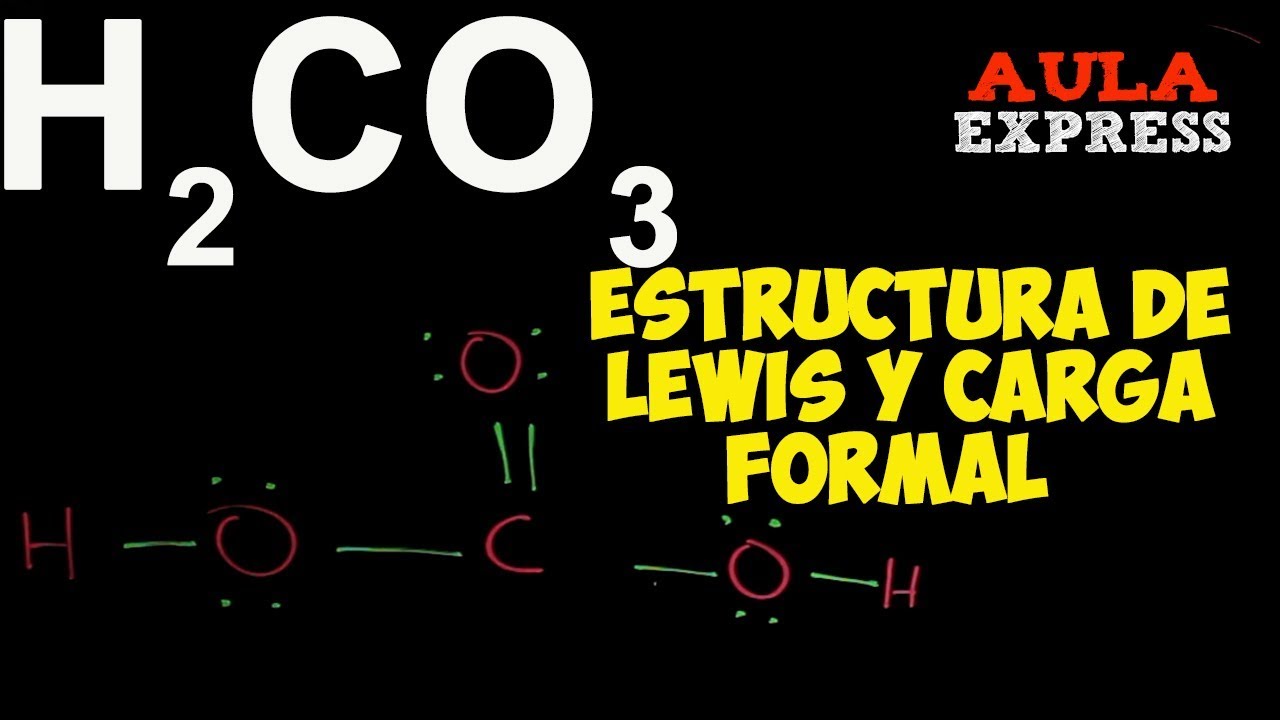
Estructura De Lewis Del H2co3 lios
A step-by-step explanation of how to draw the H2CO3 Lewis Dot Structure (Carbonic Acid). For the H2CO3 structure use the periodic table to find the total number of valence electrons.
H2CO3 Carbonic acid molecule Royalty Free Vector Image
Carbonic acid is a weak dibasic acid with a chemical formula H2CO3. It is prepared from the reaction between carbon dioxide (CO2) and water (H2O) having molar mass 62.03 g/mol and melting point -800 C. Carbonic Acid Lewis Structure Hydrogen, carbon and oxygen are three atoms present in carbonic acid.
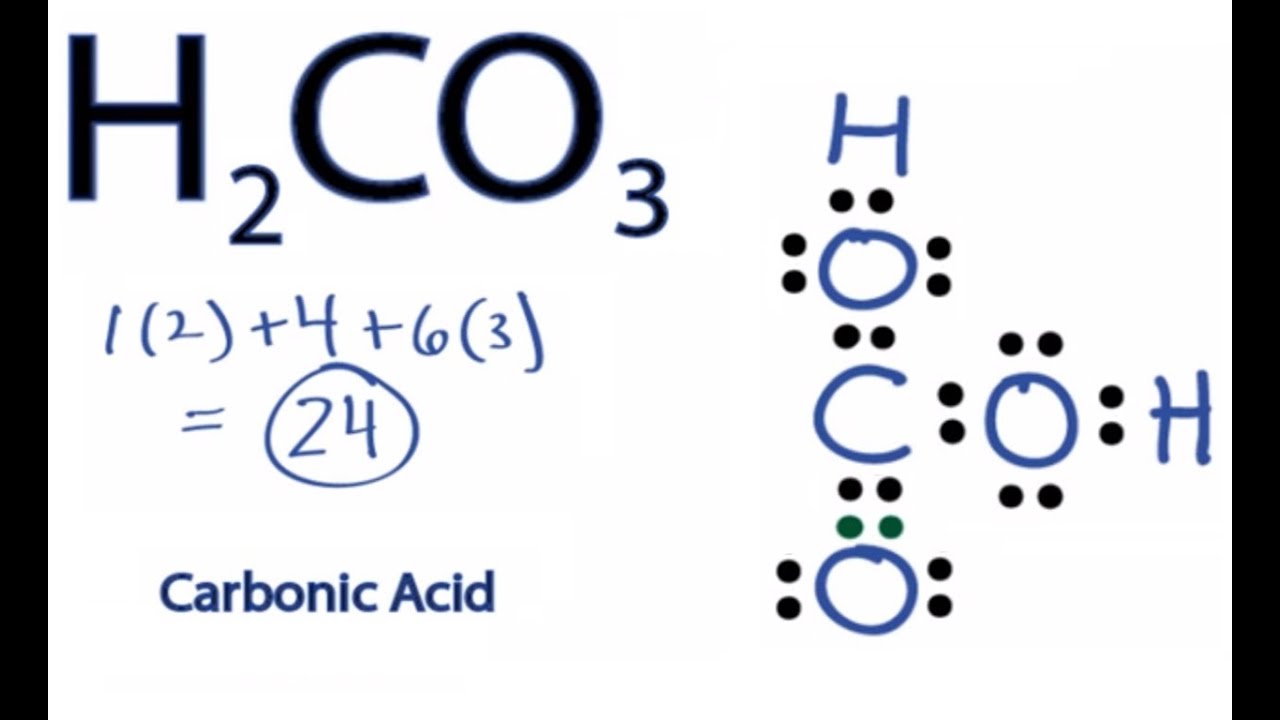
H2CO3 Lewis Structure How to Draw the Lewis Structure for Carbonic
H2CO3 lewis structure has a Carbon atom (C) at the center which is surrounded by one Oxygen atom (O) and two O-H groups. There is 1 double bond between the Carbon atom (C) & Oxygen atom (O) and the rest other atoms have a single bond. There are 2 lone pairs on all three Oxygen atoms (O).

Estructura De Lewis Del H2co3 lios
We use Lewis symbols to describe valence electron configurations of atoms and monatomic ions. A Lewis symbol consists of an elemental symbol surrounded by one dot for each of its valence electrons: Figure 7.9 shows the Lewis symbols for the elements of the third period of the periodic table. Figure 7.9 Lewis symbols illustrating the number of.
[Solved] What will be the charge of the ion formed from each of these
The lewis structure of H2CO3 contains one double bond and four single bonds, with carbon in the center, and two hydrogens and three oxygens on either side. There are two lone pairs on each oxygen atom, and carbon atom and hydrogen atom do not have any lone pair. How to draw lewis structure of H2CO3? #1 Draw skeleton #2 Show chemical bond
H2CO3 Lewis Structure, Molecular Geometry, Hybridization, Polar or
Structural Formula. H 2 CO 3. carbonic acid

Week04_02B Lewis Structure of H2CO3 (carbonic acid) YouTube
Lewis Structure of Carbonic Acid (H2CO3) The formula of carbonic acid is H2CO3. It has two H atoms, one C atom, and three O atoms. To understand the molecular formula of H2CO3, we have to observe the electronic configuration of the participating atoms and how many atoms they have in the outer shell.

[Chemistry] Lewis Structure for H2CO3 Does it matter how it looks
In the lewis structure of carbonic acid (H 2 CO 3 ), carbon atom is the center atom and there are two -OH groups. Also, there is one double bond between carbon and oxygen atoms. As some molecules. there are no lone pairs on carbon atom. From H 2 CO 3 lewis structure, we can say H 2 CO 3 is a dibasic acid.

H2CO3 Lewis Structure, Molecular Geometry, Hybridization, and MO
Carbonic acid, H2CO3 comprises of two H atoms, one C atom, and three O atoms. To understand lewis structure, first, we know basic about valance electrons, octet rule, and formal charge. The number of electrons in an element's outermost shell determines the valency of the element.

Draw the dot structure of H2CO3. Brainly.in
Lewis structure of H2CO3 (Carbonic acid) contains one double bond between the Carbon atom (C) & one Oxygen atom (O) and the rest other atoms are single bonded with each other. The Carbon atom (C) is at the center and it is surrounded by one Oxygen atom (O) and two O-H bonds. All the three Oxygen atoms have 2 lone pairs.

lewis dot structure of h2co3 is Chemistry 16239199
$\ce{H2CO3}$ exists only in very dilute solution in water, and in equilibrium with $\ce{CO2}$ and $\ce{H2O}$. If you try to make a reaction with the carbonyl group of $\ce{H2CO3}$, it will first be decomposed before any other reaction. The only exception is its reaction with the OH- ion.

Lewis dot structure of carbonic acid H2CO3 Chemistry Net
H2CO3 is one of the most known chemicals and is a chemical formula for Carbonic acid. In today's video, we help you determine its Lewis Structure by followin.

Lewis electron dot structure of carbonic acid H2CO3 YouTube
A step-by-step explanation of how to draw the H2CO3 Lewis Structure (Carbonic Acid). When we have an H (or H2) in front of a polyatomic molecule (like CO3.